Explore Our Innovative Pipeline
Pharmacology As Complex As The Diseases We Treat
Stem cells heal injured and diseased tissues through the paracrine release of exosomes and other soluble factors, collectively known as the cell’s secretome. Over 300 factors in the secretome mediate its anti-inflammatory, anti-fibrotic, pro-angiogenic, and antioxidant effects.
Our lead clinical asset, STM-01, is a neonatal cardiac progenitor cell (nCPC) in Phase 1 clinical trials in heart failure with preserved ejection fraction (HFpEF) and dilated cardiomyopathy (DCM).
Our lead preclinical asset, STM-21, is the secretome from STM-01. We are developing STM-21 for neurological conditions including amyotrophic lateral sclerosis (ALS) and diabetic neuropathy, and for burns and other inflammatory conditions of the skin.
Research and Development Pipeline
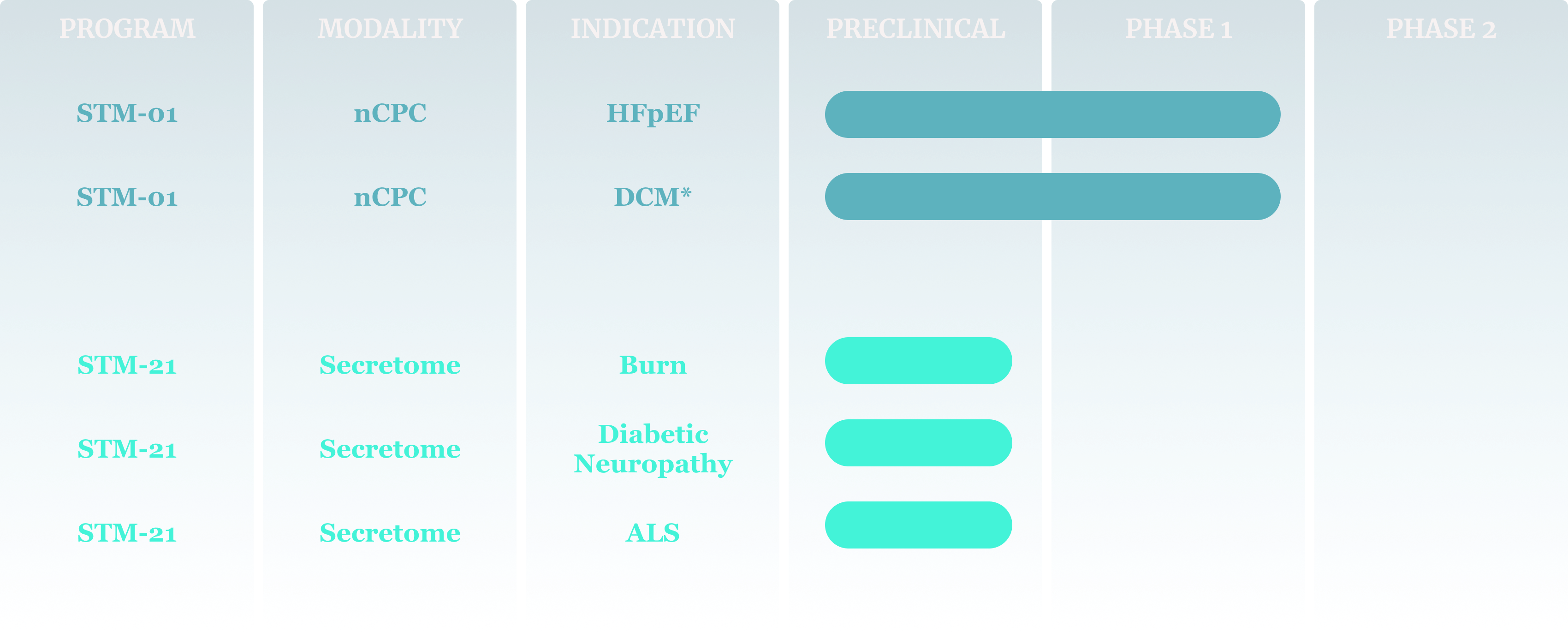
*Investigator-sponsored study at Emory/CHOA funded by the Marcus Foundation
Contact Information
Baltimore Office
323 W. Camden Street, Suite 600
Baltimore, MD 21201
Chicago Lab
400 N. Aberdeen Street, Suite 900
Chicago, IL 60642
Plano Office
7250 Dallas Parkway, Suite 400
Plano, TX 75024
